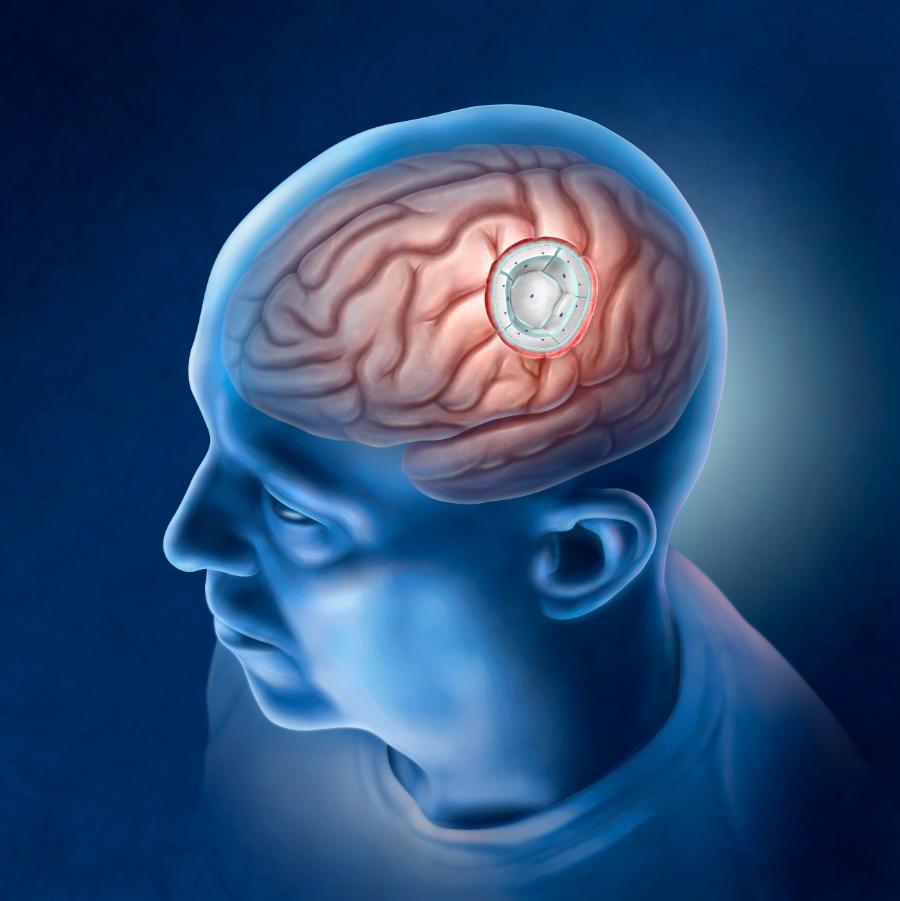
Haynes Neurosurgical Group now offers GammaTile® Therapy, which is the latest FDA-cleared device for the treatment of operable brain tumors. Brain tumors can significantly impact various aspects of your brain's functioning and early treatment of brain tumors is crucial for better patient outcomes.
Our team is excited to introduce this innovative and advanced treatment as part of our commitment to providing advanced, compassionate care to our patients.
GammaTile® Therapy is a Surgically Targeted Radiation Therapy (STaRT) designed to deliver precise radiation treatment directly to the tumor bed during surgery. GammaTile is a biocompatible, permanent collagen tile that is a source of Cesium-131 (Cs-131). It gradually releases radiation to target residual tumor cells while minimizing damage to surrounding healthy tissue.
Each tile is 2 cm x 2 cm and is 4 mm thick. Each tile contains 4 Cesium-131 (Cs-131) titanium-encased sources.
During tumor removal surgery, our skilled neurosurgeons strategically place GammaTiles® at the tumor site. The number of tiles used will depend on the size and location of the tumor, covering the tumor cavity with tiles.
These tiles immediately begin delivering a uniform radiation dose immediately, targeting any remaining cancer cells effectively.
Over time, the radiation dose is delivered, and the tiles are naturally absorbed by the body, leaving behind only inactive titanium sources.
- GammaTile® Therapy is designed to deliver 50% of the therapeutic dose within the first 10 days after surgery. This is said to help prevent residual tumor cells from replicating.2
- GammaTile® Therapy also claims to deliver 88% of the therapeutic dose within 30 days, with more than 95% of the dose delivered by 6 weeks.2
GammaTile® Therapy offers multiple benefits:
- Targeted Treatment: GammaTile® Therapy targets residual tumor cells precisely, maximizing effectiveness while minimizing damage to healthy brain tissue.
- Convenience: Administered during surgery, GammaTile® Therapy eliminates the need for repeat radiation treatments, additional hospital visits, or outpatient treatments.
- Reduced Side Effects: By delivering targeted radiation therapy, GammaTile® Therapy helps minimize side effects commonly associated with traditional radiation treatments.
Studies have shown that GammaTile® Therapy can significantly reduce treatment site recurrence in patients with recurrent meningiomas and brain metastases. Additionally, it demonstrates a potential for improved overall survival in patients with recurrent glioblastomas.5–6
A large number of patients experience fewer side effects than with other radiation treatments.1–2, 6 Postoperative side effects, such as nausea, vomiting, headache, sleepiness, neurodeficit, seizures, and skin irritation, may be experienced by some patients. However, these side effects should settle on their own as the body adjusts.
Symptoms related to radiation exposure vary depending on the radiosensitivity of the exposed tissue, the amount of radiation delivered, and the placement of GammaTile®. If you experience any unexpected effects, please contact our team immediately.
- Nakaji P, Youssef E, Dardis C, Smith K, Pinnaduwage D, Brachman D. Surgically targeted radiation therapy: a prospective trial in 79 recurrent, previously irradiated intracranial neoplasms. Poster presented at: 2019 AANS Annual Scientific Meeting; April 2019; San Diego, CA.
- Brachman D, Youssef E, Dardis C, et al. Surgically targeted radiation therapy: safety profile of collagen tile brachytherapy in 79 recurrent, previously irradiated intracranial neoplasms on a prospective clinical trial. Brachytherapy. 2019;18(3):S35-S36.
- Brachman D, Youssef E, Dardis C, et al. Resection and permanent intracranial brachytherapy using modular, biocompatible cesium-131 implants: results in 20 recurrent, previously irradiated meningiomas. J Neurosurg. 2019;131(6):1819-1828
- Rogers L, Nakaji P, Youssef E, et al. Resection and surgically targeted radiation therapy for initial salvage treatment of aggressive meningioma: results from a prospective trial. Presented at: CNS 2020 Virtual Meeting; September 30, 2020.
- Nakaji P, Smith K, Youssef E, et al. Resection and surgically targeted radiation therapy for the treatment of larger recurrent or newly diagnosed brain metastasis: results from a prospective trial. Cureus. 12(11):1-12.
- Choi M, Zabramski J. Re-Irradiation using brachytherapy for recurrent intracranial tumors: a systematic review and meta-analysis of the literature. Cureus 12(8): e9666. doi:10.7759/cureus.9666.
Indication: GammaTile is intended to deliver radiation therapy in patients with newly diagnosed malignant intracranial neoplasms and recurrent intracranial neoplasms. Side effects related to GammaTile Therapy are rare and may include radiation brain changes including necrosis. Refer to the instructions for use for a complete description of all warnings, precautions, and contraindications.